An update on newer therapies for myasthenia gravis
KnowledgeDuring a roundtable meeting for myasthenia gravis (MG) specialist nurses in October 2023, two presentations were given on MG and treatment mechanisms for the condition. With kind consent from the speakers, we are able to share the core content of those presentations here, as free access education.
Dr Channa Hewamadduma opened his presentation on some of the new and emerging therapies for MG with a brief overview of the many forms of MG, some diagnostic tests, and a quick reminder of the pathophysiology - using the figure from Howard (2017).
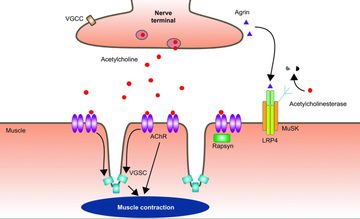
He explained that understanding the pathophysiology helps us to understand the treatment options and potential mechanisms for future targeting and helpfully mapped existing treatment avenues onto the initial pathophysiology figure.
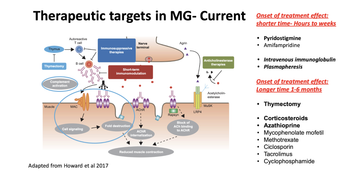
Channa noted that we need new therapies for people with MG because in up to 30% of those with MG, patients are refractory, whilst for others current treatments do not suit them - they have underlying health conditions making some treatments unwise or unavailable or experience side-effects they cannot tolerate.
Channa discussed the new therapeutic targets in MG, going into particular detail around C-5 complement inhibitors and FcRn inhibitors.
Zilucoplan, one of three new C-5 complement inhibitors was reviewed in the RAISE study, (Howard 2023). It has had favourable outcomes and has been approved by the FDA, in Japan and in Europe. The drug showed 'rapid and clinically meaningful improvements in myasthenia gravis-specific efficacy outcomes, had a favourable safety profile, and was well tolerated, with no major safety findings'. Another C-5 inhibitor, Elculizumab, used a sample of people with refractory MG (resistant to treatment). The intravenous treatment was reviewed in the REGAIN study and whilst it missed its primary endpoint was considered a positive study; similar treatment ravulizumab had similar outcomes (Vu 2022).
FcRn inhibitors block receptors to enable recycling of immunoglobulin and there are a range of clinical trials ongoing in more than five different drugs utilising this mechanism. Efgartigimod, studied in the ADAPT trial (Sacca 2023), met its primary endpoint, was found to improve health-related quality of life in patients within the first weeks of treatment, and had no significant adverse events. Rozanolixizumab, another FcRn inhibitor, also found clinically-meaningful improvements in people with generalised MG (Bril 2023).
He followed this comprehensive review with a couple of case studies, highlighting where these treatments have had a significant impact on people living with MG, and closed with a reminder than, in addition to anti-CD20 rituximab, there are a number of other B cell modulators currently in the pipeline to watch for as well.
For more information, the following references may be useful, or to dig more deeply into MG, consider attending our MasterClass in March.
Howard JF Jr. Myasthenia gravis: the role of complement at the neuromuscular junction. Ann N Y Acad Sci. 2018 Jan;1412(1):113-128. doi: 10.1111/nyas.13522. Epub 2017 Dec 21.
- Vu T, Wiendl H, Katsuno M, Reddel SW, Howard JF Jr. Ravulizumab in Myasthenia Gravis: A Review of the Current Evidence. Neuropsychiatr Dis Treat. 2023 Dec 1;19:2639-2655. doi: 10.2147/NDT.S374694

Related articles

Outstanding feedback for SMA Education Day 2025

Rare Disease Day 2025

Spinal muscular atrophy: education with impact
Raising awareness, improving outcomes
Neuromuscular Academy is the first bespoke course for healthcare professionals to receive expert training in neuromuscular conditions like spinal muscular atrophy.