Before you watch this webinar
Enhancing your learning experience begins with understanding you better. Collecting data enables us to tailor our educational content specifically for our audience. Discover more about how we handle your information in our Privacy Policy.
Event
UK stem cell transplantation in MS (the StarMS trial)
Objectives:
This webinar will review the background to stem cell transplantation in MS, a summary of the transplantation process, an overview of the StarMS trial and the current status of the trial.
At the end of the webinar, attendees can ask their questions during a live Q&A.
CPD accreditation
'UK stem cell transplantation in MS (the StarMS trial)' has been approved by the Federation of the Royal Colleges of Physicians of the United Kingdom for 1 category 1 (external) CPD credit(s). To claim the credit please email events@neurologyacademy.org.
Presentation slides
Summary
Consultant neurologist Prof Basil Sharrack opened the webinar by introducing the speakers, and explaining that Prof Jon Snowden, consultant haematologist will firstly discuss the stem cell transplantation procedure, before Basil introduces its use in people with MS, followed by Jen Petrie, clinical trial manager discusses the StarMS* trial specifically.
*The StarMS trial compares the clinical efficacy of autologous haematopoietic stem cell transplantation (aHSCT) against highly effective disease modifying therapies (DMTs) in patients with highly active relapsing remitting multiple sclerosis (RRMS).
Jon Snowden began his presentation with a short glossary lesson, which was useful in having a clear understanding as the talks went on:
- Allogeneic - refers to stem cells from a donor. A higher risk procedure with immune-based complications, often used for malignant diseases such as leukemia.
- Autologous - refers to stem cells from the patient themselves. More commonly used and safer, this tends to be used for malignant diseases such as lymphoma and autoimmune disease such as MS.
He then talked delegates through the overall process involved in autologous HSCT (fig 1) in essence, and then in slightly more detail with time frames involved (fig 2).
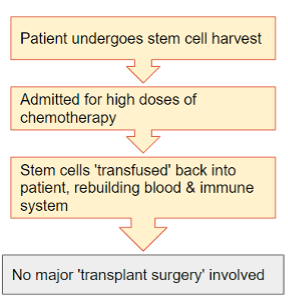
Figure 1: aHSCT process
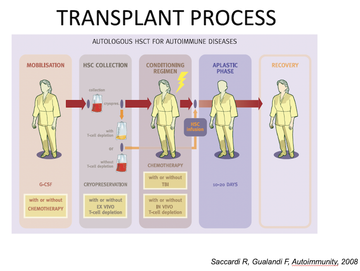
Figure 2: aHSCT process for autoimmune disease with timeframes included
Jon explained that whilst this is not a drug, there are stringent governing bodies and guidance setting organisations in place to ensure safety, efficacy, and so on, and that whilst the procedure has gained public awareness in reasonably recent years, there has been a growing evidence base since the mid-90's when the first procedure was done for a patient with autoimmune disease.
Whilst there was some skepticism and concern over the risk for stem cell transplantation in MS, the evidence base 'caught up' and there has been a considerable rise in the number of people with autoimmune systems accessing HSCT - a large proportion of these being people with MS (fig 3).

Figure 3: Number of people with MS accessing aHSCT annually (1995-2020)
Jon shared that some countries have had much more interest than others, chiefly UK, Italy, Sweden, Poland and Spain and pondered whether the funding element might be at play here; the UK has been funding this treatment for around a decade now.
Jon discussed 'the mechanisms of action', detailing that no single cell is targetting, but that a general 'debulking' of inflammation occurs as well as 'resetting the dysfunctional immune system' and various studies have found that the treatment may have a longer-term effect of re-diversifying the T-cell receptor's repertoire (Delamarre 2015) and increasing T-cell regulatory cells.
Jon closed by highlighting that there is a significant cost associated with HSCT, and noted that his colleagues on the webinar would consider this cost/benefit to both the patient and the NHS in more detail.
Basil Sharrack began by sharing the cost of various high-efficacy treatments in MS, noting that the costs cited are for the treatment only, not the monitoring and administration required to provide them (fig 4). He voiced that, as a one-off treatment, it is cost-effective, if it is efficacious.

Figure 4: Cost of MS-treatments by comparison
Basil shared a short case study of a young lady with RRMS who was progressing very quickly and not responding to other treatments, who underwent stem cell treatment very successfully; however, he was clear that HSCT is not for everyone and that choosing patients who are suitable must be done carefully.
'This treatment is a sledgehammer and we cannot crack every nut with it - we have to choose our patients carefully.'
Basil presented data of 2 years NEDA post-treatment in MS across a range of different treatments, including placebo, fingolimod, natalizumab, alemtuzumab, cladribine, ocrelizumab and aHSCT and highlighted that, with aHSCT, the worst result achievable is a NEDA of around 70% which is higher than the best result on any other treatment. However, the aHSCT results have not come from clinical trials, whilst the various disease-modifying therapies (DMTs) all have.
This prompted clinical trials - the first of which was MIST (Burt 2019). This compared two groups of people with MS on either a DMT or aHSCT.
- Referring to time to disease progression post-treatment, at a median of 2 years, 34 people in the DMT group had experienced disease progression, whilst only 3 in the aHSCT group had.
- Mean EDSS scores increased in the DMT group (3.31 to 3.98) but decreased markedly (3.38 to 2.36) in the aHSCT group, which is heretofore unheard of in clinical trials for MS treatment.
Basil noted some of the limitations of this study - chiefly that aHSCT needed to be trialed with other types of MS.
He then shared a Canadian study trialing aHSCT in 24 patients with 'aggressive MS' (Atkins 2016) where findings were 'unparalleled to anything else':
- Every patient experienced NEDA
- There was no future relapse following treatment
- Some people's EDSS reduced massively, but others continued to worsen - whilst aHSCT can 'switch the illness off', it depends on the person as to whether their disability will be reversed or not.
Basil cited a wider evidence base having found similar results with the best results in RRMS and younger people rather than those with more progressive MS although it has been used to good effect in those with aggressive or progressive MS as well.
Basil mentioned mortality, highlighting that in the early cases of treatment in 1990's there was almost a 7% risk of mortality but that this has dropped substantially - in MIST, mortality was zero, and the usual quoted mortality is 1%. Whilst very low, Bail was clear that patients need to be counselled very strongly about this.
Basil then shared guidelines from the ABN on aHSCT and the NHS algorithm, where it is stated as a third-line treatment for those who have failed on other treatments. The EBMT recommends it as a treatment option for a wider variety of patients (Sharrack 2019).
He explained that in Sheffield, they follow the EBMT guidelines and offer aHSCT to patients with inflammatory active disease who have failed to respond to a DMT, putin them forward for clinical trials is possible, but offering them the treatment on a compassionate case-based basis if they do not meet any trial inclusion criteria.
Jen Pitrie continued from where Basil left off, presenting the StarMS trial taking place in Sheffield currently, which compares aHSCT with three of the most recently developed high-efficacy DMTs available in MS: alemtuzumab, cladribine and ocrelizumab.
Jen provided an overview of the study(fig 5) and noted that whilst there were delays in setting up due to the pandemic, they began with one person in the trial last September and a further two going through the screening process.
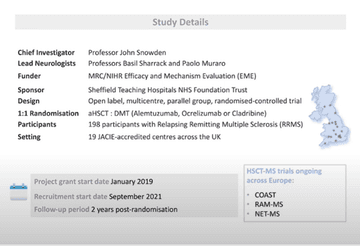
Figure 5: StarMS study overview
She explained the dual aims of the trial are to compare the aHSCT efficacy against those new DMTs that were not included in the MIST trial, to establish whether it has an acceptable safety profile and to advance existing understanding of the mechanisms of efficacy.
With the core objective of NEDA at 2 year post-treatment, Jen explained the measurements that will be used, and the additional aims including those around quality of life. Jen also explained the three optional substudies and the collection of bloods and CSF serum for use in future studies.
Jen summarised key eligibility criteria, noting that the trail has strict inclusion and exclusion criteria (fig 6) and went on to explain the different treatment arms and how these are chosen - with the local clinician and patient making a shared decision based on what is best for them.

Figure 6: StarMS study eligibility criteria
References
Atkins et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet. 2016 Aug 6;388(10044):576-85. doi: 10.1016/S0140-6736(16)30169-6. Epub 2016 Jun 9. PMID: 27291994.
Burt et al. Effect of Nonmyeloablative Hematopoietic Stem Cell Transplantation vs Continued Disease-Modifying Therapy on Disease Progression in Patients With Relapsing-Remitting Multiple Sclerosis: A Randomized Clinical Trial. JAMA. 2019 Jan 15;321(2):165-174. doi: 10.1001/jama.2018.18743. PMID: 30644983; PMCID: PMC6439765.
Delemarre et al. Autologous stem cell transplantation aids autoimmune patients by functional renewal and TCR diversification of regulatory T cells. Blood (2016) 127 (1): 91–101. https://doi.org/10.1182/blood-2015-06-649145
Sharrack, B., Saccardi, R., Alexander, T. et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant 55, 283–306 (2020). https://doi.org/10.1038/s41409...
Encouraging excellence, developing leaders, inspiring change
MS Academy was established in 2016 and in that time has accomplished a huge amount with exciting feedback demonstrating delegates feel inspired and energised along their personal and service development journeys. The various different levels of specialist MS training we offer are dedicated to case-based learning and practical application of cutting edge research.


