Before you watch this webinar
Enhancing your learning experience begins with understanding you better. Collecting data enables us to tailor our educational content specifically for our audience. Discover more about how we handle your information in our Privacy Policy.
Event
Debate session – Should ocrelizumab and cladribine be treated in the same way as alemtuzumab in terms of risk of COVID-19?
Our sponsor

This activity has been supported by sponsorship from and Roche. The sponsor has had no control over the educational content of this activity.
Presentation slides
Klaus slides
David's slides
Summary
The webinar was hosted by Profs Klaus Schmierer & David Baker. The debate session discussed whether ocrelizumab and cladribine ought to be treated in the same way as alemtuzumab in terms of risk during the COVID-19 pandemic.
This summary covers:
- Voting results
- Yes…
- No…
- Discussion and questions
Votes on the subject
Should ocrelizumab and cladribine be treated in the same way as alemtuzumab in terms of risk of COVID-19? Join the debate tomorrow with Prof's Klaus Schmierer & David Baker, Barts and The London School of Medicine and Dentistry https://t.co/h5aVmi6QiW @ACNRjournal @TheUKMSSNA
— Neurology Academy (@TheNeuroAcademy) March 30, 2020
Opening the debate, before any arguments had been made or discussion held, the vote was 67% no, 33% yes.
Closing the debate, following the proposals, discussion and question opportunities, the vote was 74% no, 26% yes.
Yes…
Prof Klaus Schmierer was first to propose his argument that ocrelizumab and cladribine should be treated the same way as alemtuzumab in terms of risk, during the COVID-19 pandemic.
Beginning with an background to the virus, Klaus noted the passing of the virus from horseshoe bats (as with SARS-CoV) to humans via civets although noted that other intermediaries such as turtles or pangolins are being discussed (3.17), and detailed that, from its onset in Wuhan, China in December 2019, it has affected 723,000 people, and caused 34,000 fatalities (a death rate of 1-5%, compared with that of influenza which has a death rate of less than 1%). Klaus highlighted a variance from SARS in that asymptomatic people can still transmit the virus with COVID-19 whereas only symptomatic people can transmit SARS-CoV, and showed the incremental rates of the virus’s spread across the globe.
Should ocrelizumab and cladribine be considered a higher risk to alemtuzumab, or conversely alemtuzumab be considered a lower risk to ocrelizumab and cladribine (6.59)? Klaus chose to take a practicing clinician’s viewpoint. Highlighting responses to a recent MS Academy survey on how COVID-19 has affected practice (7.48). Klaus noted that a sub-group of the Association of British Neurologists have recently changed the regularity of laboratory follow up testing for both alemtuzumab and cladribine from monthly to every three months to help services cope with the burden of demand (8.42).
Arguments:
- Keep it simple.
- This is a medicolegal issue. We expose ourselves to risk if we do not follow the advice given by our designated bodies (9.37).
- We should follow the available guidance on disease-modifying treatment (DMT) use for MS during COVID-19 such as published by the ABN (12.31)
- Whilst we can see that 97% of people had no more than grade 2 lymphopaenia after a course of cladribine (10.59) there are a small number of patients who do go on to develop grade three or four; with reduced blood monitoring we may miss these patients.
- There is no need to take additional risk during this crisis, particularly as the Natalizumab eligibility criteria have been relaxed by the ABN, giving more options.
- Klaus added and then questioned the use of ‘always’ in the motion ‘should ocrelizumab and cladribine always be treated in the same way as alemtuzumab?’ arguing that, regardless of pandemic, patients’ individual lymphocyte counts should be the key to this question rather than the drug itself, as lymphocyte recovery varies from patient to patient.
No…
Prof David Baker then proposed that ocrelizumab and cladribine should not be treated the same way as alemtuzumab in terms of risk, during the COVID-19 pandemic.
He was clear that he comes to the debate from the scientific angle, being a neuroimmunologist rather than a neurologist, and highlighted his starting point was also the revised ABN guidelines. As an introduction to his argument, he asked whether the guidelines are based on opinion or evidence. He noted that the amends to the guidelines have come about as a result of COVID-19 which is largely thought not to be neurotropic (16.50); then shared that there is some evidence to suggest that one of the issues could be neurotropism of the virus (see Dr Sharmilee Gnanapavan’s webinar on CSF analysis of COVID-19). And finally, he highlighted that the three DMTs, ocrelizumab, cladribine and alemtuzumab, are all different drugs to one another (17.01) and ought not to be grouped two to one.
Querying the logic behind the ABN revised guidelines (17.17), David compared the ABN’s argument that alemtuzumab and cladribine be treated the same, with Australia’s guidance which clearly states that they be treated differently owing to the non-immunosuppressant nature of alemtuzumab and the distinctly lower risk of infection (18.28). David used the argument of varying sets of ‘evidence’ being presented on Twitter, all from reputable sources but all at odds with each other (19.42).
The true question to ask is ‘what do we know about the immune response to COVID-19?’ (see figure 1).
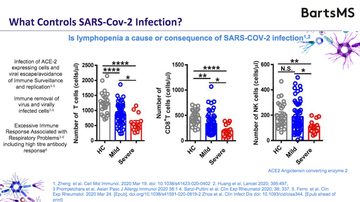
Figure 1: The process from infection to immune response from COVID-19 (slide 8)
Whilst there is limited knowledge about COVID-19, David argued that a great deal is known about SARS-CoV (20.52) and discusses what is known here. Detailing experimental evidence from giving monkeys coronavirus (21.26), and then from a patient (22.27) he highlights that whilst immunity kicks in within the first few days, antibodies are not made until the virus has almost run its course, suggesting that it may not be the antiviral response as important as fighting the virus initially. He then details evidence that it may be macrophages which initially form resistance to the virus (23.46).
David then looked at the DMTs, suggesting that all three – alemtuzumab, cladribine and ocrelizumab – are largely the same in terms of their ability to inhibit relapse, based on their capacity to inhibit B cells or memory B cells. However, the ABN’s guidance seems to focus on their ability to inhibit T and B cells and David notes that cladribine actually causes B-cell depletion with some T-cell depletion meaning it’s more similar to ocrelizumab. He also notes that, regarding social distancing, cladribine may have some advantages over other DMTs.
David then examines the differences and similarities in detail with regard to their cell depletion and the likelihood of causing grade 3 or 4 lymphopaenia (25.44) (alemtuzumab in 80% of cases versus cladribine in 3%), the rapidity of B-cell recovery (26.35), and the impact on T-cells (28.05 and 28.47). Research suggests that a low level of lymphopaenia puts a person at around twice the likelihood of developing an infection.
In summing up the evidence (29.34):
- alemtuzumab puts a person at a higher risk of infection compared to cladribine or ocrelizumab
- Efficacy is relatively similar because the working mechanism is the same, causing long-term depletion of memory B-cells.
- The advantage of alemtuzumab and cladribine is that the person does not maintain therapy – alemtuzumab takes one month to disappear, cladribine just one day to disappear, whatever cells remain can begin to respond to any infection.
- Immature B-cell depletion suggests the infection risk but the T-cell extra depletion contributes to that risk, meaning it is clear that alemtuzumab has a greater risk than cladribine or ocrelizumab.
- The best way to avoid infection is to keep outside of hospitals, meaning accessing treatments which are effective, can be delivered away from hospitals and do not have excessive monitoring.
It is clear from the science that there is a definite distinction between ocrelizumab, alemtuzumab and cladribine and so the answer to ‘Should ocrelizumab and cladribine be treated in the same way as alemtuzumab in terms of risk of COVID-19?’ must be ‘no’.
Discussion and questions
If you are covid-positive, when is the moment to restart high efficacy therapy? (32.54)
When you have cleared the virus, and are symptom free. Lymphopaenia results are a good suggestion too. David noted that the plasma cells in bone marrow form immunity as well as memory-B cells and that there is evidence that childhood immunity is retained when receiving treatments.
Are you suggesting that cladribine may be an option as treatment during this time? (35.25)
One could make the argument; if you are self-isolating or practicing social distancing for three months, it could perhaps work, but it would be a high risk strategy. As NHS England have relaxed the eligibility criteria for natalizumab during the pandemic, that would be the safer choice.
If you have a new patient with very active RRMS would you consider ocrelizumab for initial treatment right away? (36.38)
Klaus:
No, I would start them on natalizumab and consider switching it after six months.
If there’s a vaccine for COVID-19 will patients respond equally in any of the treatments? (37.19)
David:
Probably not. There is data with alemtuzumab for a vaccine response; 60-62% of people make an anti-alemtuzumab response. Even with no T cells and no B cells you can make an antibody. There is data (unpublished paper) on ocrelizumab can make an anti-vaccine response but it is blunted. There isn’t any data for cladribine but theoretically the immature B-cells start to recover and you have T cells at that point so there is no reason why a vaccine response is not possible.
Regarding lessening hospital attendance requirements with some drugs and risk of HAI (autoimmunity), isn’t this a significant factor? (41.22)
Klaus:
David’s slides showed the extended period that ocrelizumab works and perhaps pregnancy is the model example that the drug works beyond its dosing interval. There is phase 2 data supporting this. It is a good option to defer ocrelizumab by three months and then resume treatment.
David:
It is very clear that once depleted, the memory B cells stay depleted for a very long time compared to the other DMTs.
Would you test patients for COVID-19 before starting them on a DMT? (43.28)
It would be ideal to do that, but the availability of testing means that is unlikely. 50% of people seem to be asymptomatic based on the Icelandic data; knowing if someone has had COVID-19 would be useful before starting on a treatment.
If someone has had their first dose of cladribine, then caught coronavirus – would they have to stop or delay the second week of administration? (44.26)
Klaus:
As a team we have discussed continuing treatment in the face of the pandemic and have agreed to continue; however, if I knew that the person was positive for COVID-19 then no, I would not treat them with the second dose; I would move to something safer then retreat.
David:
With cladribine, we know it is a cell killer, a lot of the benefit is from the first dose.
Due to the IFN viral mechanism, could we make a switch from cladribine to IFN to protect patients in the meantime? (47.38)
David:
There are trials with type-1 interferon going on currently. These trials that will give us answers to this sort of question in the next couple of months.
Is taking the first course of cladribine at the moment safer than taking the second course (2nd year) because the prevalence of lymphopaenia is different? (49.47)
Klaus:
Not as strong evidence for that anymore so there is not a strong argument in favour of that.
Prof Baker had a slide noting for ocrelizumab ‘immature B-cells depleted permanently’ – can you explain more? (50.41)
David:
it maintains the B-cell pool at a lower level whilst on treatment. Ocrelimzumab is still depleting 5 or 6 months later because it is such a high dose.
How do you feel about natalizumab extended interval dosing (EID) to reduce the risk of COVID-19 without strong evidence of efficacy of EID? (51.53)
Klaus:
There was good evidence in a recent paper.
If a patient has had one cycle of ocrelizumab, would you delay the second (3 months) infusion, or would you only delay if after the second cycle? (52.26)
Klaus:
It’s a judgement call; I would probably complete the first full cycle and then ask people to self-isolate for three months.
Were you registered on this course?
Log in to access resources..
LoginOur sponsor

This activity has been supported by sponsorship from and Roche. The sponsor has had no control over the educational content of this activity.
Encouraging excellence, developing leaders, inspiring change
MS Academy was established in 2016 and in that time has accomplished a huge amount with exciting feedback demonstrating delegates feel inspired and energised along their personal and service development journeys. The various different levels of specialist MS training we offer are dedicated to case-based learning and practical application of cutting edge research.






