Before you watch this webinar
Enhancing your learning experience begins with understanding you better. Collecting data enables us to tailor our educational content specifically for our audience. Discover more about how we handle your information in our Privacy Policy.
This area of the website is reserved for healthcare professionals as it contains information created for a healthcare professional audience.
Event
Vaccinations in people with multiple sclerosis in the era of highly effective DMTs
Our sponsor

Objectives:
- To review the latest guidelines on vaccination in MS from ECTRIMS/EAN
- To discuss what vaccinations are required and when in people starting high-efficacy treatments including pneumococcal, Zoster and COVID-19 vaccination
- How to approach vaccine hesitancy and apathy in people with MS
- The immunosuppressed traveller - vaccinations and preventing infections in people with MS during overseas travel
Topics for discussion:
Infections and infection related morbidity and mortality are higher in people with MS than the general population. Vaccination is one of the most effective ways to prevent infections in people with MS but decisions regarding vaccination have become more complex in the era of widespread high-efficacy drug therapies in MS. This webinar will discuss the latest UK and international guidelines on vaccination (including pneumococcal, Zoster and COVID-19 vaccination), approaching patients hesitant to have vaccinations, and vaccinations in the immunosuppressed traveller.
Summary
Vaccinations in people with multiple sclerosis in the era of highly effective DMTs
Disease modifying therapies (DMT) have mechanisms of actions that involve alterations in lymphocyte trafficking, lymphocyte depletion, and the disruption of lymphocyte replication.
This puts patients who are taking the drugs at an increased risk of developing infections, and experiencing a more severe course, making vaccination an important consideration.
The European Committee for Treatment and Research In Multiple Sclerosis (ECTRIMS) and European Academy of Neurology (EAN) published a joint consensus on vaccination in people with MS in July 2023.
One of the key messages, said Dr Claire McCarthy, consultant neurologist at Cambridge University Hospitals NHS Foundation Trust, is that vaccines are safe for people with MS. "We can now be confident that the benefits of vaccination far outweigh the risks," she added.'
Guideline summary
Common vaccinations, such as for flu, tetanus, or bacille Calmette-Guerin (BCG) do not seem to increase the risk of MS exacerbation or disability progression, the document states.
Inactivated vaccines can be safely used in people receiving DMTs. Live vaccines can be used in those not on DMTs, and those on interferons or glatiramer acetate (GA). In all other cases, they should be avoided
The statement also explains that protection after vaccination is similar across the general population and people with MS both receiving and not receiving interferons or GA.
People on dimethyl fumarate (DMF), teriflunomide, or natalizumab may produce lower levels of antibodies than non-treated patients, but still achieve sufficient seroprotection. The same is true of those taking sphingosine-1-phosphate modulators and anti-CD20s, though their seroprotection can be reduced. There is limited data on protection in those receiving alemtuzumab or cladribine. The therapies' mechanism of action would suggest reducedn seroprotection, write the authors.
"The recommendation here is to counsel patients about diminished protection against infection and how to follow other protective strategies," said Aoife Shields MS pharmacist at the National Hospital for Neurology and Neurosurgery.
Preparedness pays, she added. The consensus guideline iterates the importance of considering immune status, discussing the risks, and recommending early vaccination at the time of diagnosis.
She also highlighted a table within the document which lists the recommending safety intervals between drug suspension and live vaccine administration:
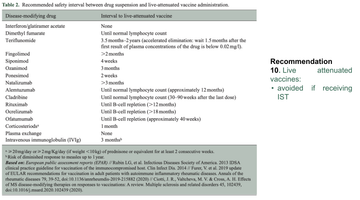
Variations in guidance
There is some variation between the guidelines and what is advised in uk practise, which must comply with the Immunisation Against Infectious Disease green book:
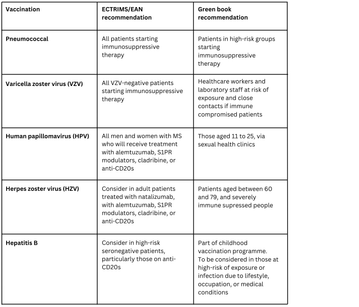
The ECTRIMS/EAN consensus guideline also includes guidance on specific sub-populations, such as paediatrics, pregnant women, the elderly, and international travellers.
For children, national vaccination guidelines apply and, ideally, all should be completed before they start an immunosuppressive therapy. Non-vaccinated children should follow a catch-up programme. "The same precautions that apply to adults apply to children," said Aoife.
In pregnancy, the guideline recommends a complete review of vaccination status, and that women receive an inactivated flu vaccine, regardless of trimester, at the start of influenza season. The document also advised diphtheria, tetanus, and pertussis (Tdap) vaccination between weeks 29 and 36.
Clinicians are also advised to measure CD-19 positive B-cells in newborns who have been exposed to anti-CD20s in utero. The administration of live vaccines should be delayed until levels have recovered.
All vaccines other than that for yellow fever are considered safe during breastfeeding.
Older people should be informed of the risk of severe infections and their altered immune response. In general, the guidelines states that vaccine strategy in terms of timings, vaccines and precautions, is the same as that of the adult MS population. They should, however, received the flu virus, pneumococcal, and inactivated HZV vaccination annually.
The ECTRIMS/EAN paper also discusses international travellers. It says that all patents can receive inactivated travel vaccines. Live vaccines such as yellow fever, oral typhoid, dengue,
varicella and measles, mumps and rubella, however, are contraindicated in those on immunosuppressive therapy.
Early discussions, with vaccine experts in conjunction with the MS team if possible, are advised. Immunisations should ideally be started two to three months before departure. The guidelines also suggests post-vaccination serology to guide the need for booster doses.
Vaccine hesitancy
Vaccine hesitancy is a common issue MS teams face, particularly around the COVID vaccine.
Mhairi Coutts, MS specialist nurse at NHS Ayrshire and Arran, said there was now a lot of evidence teams can share with patients regarding the safety of vaccines.
She also outlined a case study of a 23-year-old woman, diagnosed in May 2022, who was convinced her MS had been triggered by the COVID vaccine. She opted to go ahead with ofatumumab, but refused to have any more vaccines.
"Thankfully her screening bloods were fine. Her disease was very active, so following discussions with the wider team, we decided to go ahead with treatment but continue to counsel her," said Mhairi, adding that the patient was made fully aware of the risks.
"You can try gentle persuasion and exploring their fears, but some people have very strongly held beliefs and you can't always shake those," said Claire. In this case, the panel agreed, minimising risk as much as possible is key.
Otero-Romero, S., Lebrun-Frénay, C., et al. (2023). ECTRIMS/EAN consensus on vaccination in people with multiple sclerosis: Improving immunization strategies in the era of highly active immunotherapeutic drugs. Multiple Sclerosis Journal, 13524585231168043.
UK Government. Immunisation against infectious disease. (2020). Available at: https://www.gov.uk/government/...
Last accessed: 28th February 2023.
Our sponsor

CPD accreditation
'Vaccinations in people with multiple sclerosis in the era of highly effective DMTs' has been approved by the Federation of the Royal Colleges of Physicians of the United Kingdom for 1 category 1 (external) CPD credit(s).
Please note CPD Federation approval does not include satellite symposia sessions.
Encouraging excellence, developing leaders, inspiring change
MS Academy was established in 2016 and in that time has accomplished a huge amount with exciting feedback demonstrating delegates feel inspired and energised along their personal and service development journeys. The various different levels of specialist MS training we offer are dedicated to case-based learning and practical application of cutting edge research.


