Fingolimod safety considerations in real-world practice: fingolimod-associated macular oedema (FAME)
Fingolimod is a first-in-class, sphingosine-1 phosphate (S1P) receptor modulator, and was the first oral disease modifying treatment (DMT) licensed for use in multiple sclerosis (MS)1.
Fingolimod has been available for use in the UK since 2012. In Scotland, it is licensed for use as a single DMT in highly active relapsing remitting MS (RRMS), for patients who meet the following criteria2:
- Patients with high disease activity despite treatment with at least one disease modifying therapy.
or
- Patients with rapidly evolving severe relapsing remitting multiple sclerosis defined by two or more disabling relapses in one year, and with one or more gadolinium enhancing lesions on brain MRI or a significant increase in T2 lesion load as compared to a previous recent MRI.
Within NHS England patients are required to have failed on a first-line DMT before being eligible3.
Efficacy in clinical trials
The pivotal trials, upon which clinical efficacy and safety of fingolimod for RRMS was based, included TRANSFORMS4 and FREEDOMS5, were published in 2010. FREEDOMS II (2014) was undertaken in order to provide further safety data on fingolimod, particularly with regard to cardiac side effects6.
TRANSFORMS, compared low (0.5 mg) and high (1.25 mg) dose fingolimod with IM interferon beta (Avonex®) over 12 months in 1,292 participants. FREEDOMS was a randomised double-blind placebo-controlled trial conducted over 24 months, including 1,272 participants, which also including two treatment arms with low and high dose fingolimod. The primary outcome in both was annualised relapse rate (ARR).
In TRANSFORMS the ARR for the fingolimod arm was 0.25, a 52% relative reduction compared with Avonex®. In FREEDOMS, low dose fingolimod resulted in an ARR of 0.21, compared with 0.54 on placebo – a reduction in relapse rate of 55%. High dose fingolimod resulted in a reduction in relapse rate of 38% in the TRANSFORMS study, but was associated with a higher rate of adverse events. FREEDOMS II reported similar efficacy results and safety outcomes were as anticipated with the now licensed 0.5 mg dose.
Mechanism of action
Sphingosine-1 phosphate is a cell signalling molecule involved in the regulation of cell growth, apoptosis, and immune cell trafficking. S1P acts via five different cell surface receptors (S1PR 1-5).
S1P receptor types 1-3 are widely expressed; receptor type 4 is found on lymphoid tissue, and S1P receptor type 5 is expressed within the central nervous system (CNS). B and T lymphocytes predominantly express S1P receptor subtype 1, which is of particular importance in lymphocyte egression from lymph nodes7.
Fingolimod alters trafficking of both naïve and antigen-activated lymphocytes from secondary lymphoid tissue, thymus and bone marrow to produce a relative lymphopenia. It does this via two non-specific mechanisms – an agonistic action on S1P receptors 1,3,4 and 5, and a functional antagonistic action against the SIP1 receptor. The cumulative effect of these actions is the internalisation of the S1P1 receptor within lymphocytes, thereby resulting in the transient retention in lymph nodes.
The reduction in circulating lymphocytes is dose dependent. Within the first week of treatment, circulating lymphocyte count falls by 20-30%. At maintenance there is a reduction of approximately 70%. The half-life of fingolimod is between six to nine days.
The non-selective mode of action of fingolimod may produce unwanted additional effects, for example bradycardia. Combined analysis of FREEDOMS and TRANSFORMS showed a mean decrease in heart rate by eight beats per minute, and an incidence of first degree atrioventricular block of 4.7%. This is thought to occur as a consequence of fingolimod’s transient agonistic effects on S1P1 receptors within atrial myocytes7.
Fingolimod-associated macular oedema (FAME)
Fingolimod was originally evaluated as a treatment for renal transplant rejection, at doses 10 times higher than now prescribed in MS. In renal transplant trials, macular oedema was noted to be twice as prevalent in patients receiving fingolimod compared to those on placebo (28% in diabetic patients and 4% in non-diabetic patients). All subsequent clinical trials involving fingolimod have therefore implemented screening for macular oedema8.
Macular oedema, from any cause, is thought to occur as a result of breakdown of the blood-retinal barrier (BRB). Patients typically present with central vision loss, blurred vision and eye pain. The specific mechanism by which fingolimod causes a breakdown of the BRB is not known however. S1P receptors 1 and 3 are involved in regulation of endothelia and epithelial barrier functions, and the action of fingolimod at these receptors may interfere with barrier integrity.
The incidence of macular oedema from the TRANSFORMS and FREEDOMS trials was 0.3% for 0.5 mg fingolimod, and 1.2% for the 1.25 mg dose, and the vast majority of cases occurred within three to four months of starting the study drug4,5. Almost three quarters of cases were unilateral and most patients (68%) presented with typical symptoms. In 84% of patients macular oedema resolved on discontinuing fingolimod. The most common risk factors identified from these studies were a history of diabetes mellitus and increasing age.
The manufacturer of fingolimod (Gilenya®) has recommended ophthalmological evaluation is undertaken at three to four months after treatment initiation9. Further, it is recommended that multiple sclerosis patients with diabetes mellitus or a history of uveitis undergo an ophthalmological evaluation prior to initiating therapy and have follow-up evaluations while receiving therapy.
It is recommended that Gilenya be discontinued if a patient develops macular oedema. The decision on whether or not Gilenya therapy should be re-initiated after resolution of macular oedema needs to take into account the potential benefits and risks for the individual patient.
Additional adverse effects
Within the trials, the overall incidence of infections was similar across fingolimod, placebo and IFN-β1a arms; however, herpes zoster, influenza and lower respiratory tract infections occurred at higher rates in participants taking fingolimod. A three-fold increase in liver transaminases was also noted in approximately 10% of study participants taking fingolimod. Additional common adverse effects include headache, hypertension, cough, dyspnoea, back pain, headache, and diarrhoea4–6.
Primary objective
- Ascertain whether patients commenced on fingolimod in our centre were adequately screened for macular oedema within 120 days after initiation, or before treatment if high-risk, as advised by the manufacturer.
Secondary objectives
- Document the proportion of patients developing macular oedema with fingolimod
- Document the proportion of patient stays extended due to cardiac abnormalities
- Document the proportion of patients developing significant infections within the first year of treatment with fingolimod
- Document the proportion of patients developing new malignancies within the first year of treatment with fingolimod.
Methods
A retrospective analysis of electronic medical records was undertaken in our regional tertiary neurology centre. The Institute of Neurological Sciences in Glasgow is the neurosciences referral centre for the West of Scotland, covering a catchment area of 2.5 million people. All patients commencing fingolimod are admitted to our day investigation unit for first-dose cardiac monitoring, save a small proportion where this is undertaken in their local hospital in Lanarkshire.
Using admissions records, we identified all patents commenced on fingolimod in our centre between May 2016 and May 2017. Each NHS patient in Scotland is assigned a unique identifier, the Community Health Index (CHI) number, which is included in admissions records and hence permits access to their electronic medical records including GP referrals, laboratory results and all secondary care correspondence. Additionally, the electronic portals are linked in the West of Scotland such that regional records are available centrally and vice versa, permitting access to all follow-up correspondence even for patents located out with our tertiary centre. Additionally, a contemporary electronic prescription, from GP records, is accessible to view current and recent medications patients have been prescribed.
Patients admitted for re-initiation of fingolimod after a break in treatment were not included.
Factors predisposing to fingolimod-associated macular oedema (FAME) and important other safety considerations were identified from review of relevant literature.
Data were entered into a Microsoft Excel® (2010) spreadsheet and analysed within this. The following information was collected:
- Age
- Sex
- History of exposure to drugs predisposing to macular oedema (diabetes treatments, Tamoxifen and topical prostaglandin agonists e.g. Latanoprost)
- History of conditions predisposing to macular oedema (diabetes, uveitis, cataract surgery, glaucoma, retinal choroidal disease and retinal vascular disease)
- DMT switched from (if fingolimod not used first-line)
- Date fingolimod started
- Presence of baseline ECG abnormalities
- Need for extended admission due to cardiac issues
- Date of referral to ophthalmology
- Date of ophthalmology review for macular oedema
- Duration of follow-up since fingolimod initiation
Descriptive statistics were generated, with the primary outcome being time between fingolimod initiation and ophthalmology assessment, the aim being less than 120 days (four months), as outlined above. Proportions were calculated for other safety considerations, as per our secondary objectives.
Results
Forty patients were commenced on fingolimod in our centre between May 2016 and May 2017. Mean age was 39 years (SD 10.9) with a range between 19 and 60 years. The majority were female (32, 80%) in keeping with the usual demographics of patients with RRMS. No patients were on medications predisposing to macular oedema but one patient (2.5%) had diabetes. Duration of available follow-up correspondence was at least six months from fingolimod initiation, with a mean of 351.8 days (SD 98.2), range 183-527.
Screening for macular oedema
All patients were referred and seen by ophthalmology to screen for macular oedema. The patient with diabetes was referred and appropriately screened prior to commencement of fingolimod. All patients underwent optical coherence tomography (OCT) as part of the ophthalmological assessment.
Mean time from fingolimod initiation to ophthalmology assessment was 124.8 days (SD 46.8, range 28-238). Two patients did not attend their first appointed ophthalmology assessment: excluding these patients (duration from fingolimod initiation to ophthalmology assessment 182 and 199 days respectively) reduced the mean duration of the cohort to 121.4 days (SD 45.4). Overall, however, 25 patients (62.5%) waited more than 120 days between fingolimod initiation and ophthalmology screening for macular oedema.
In considering reasons for delays, the mean duration between fingolimod initiation and ophthalmology referral was 15.6 days (SD 15.8) but up to 50 days in one case. Two patients were ‘pre-referred’ prior to fingolimod dosing notably, although one still waited beyond 120 days. Generally, however, the greatest delay was awaiting the ophthalmology appointment, with mean 109.3 days (SD 41.6) from referral, but up to 230 days in one case and 17 patients (42.5%) waiting more than 120 days for this alone, as outlined in Figure 1.
Macular oedema was found in two patients (5%), neither of whom had a predisposing condition or drug therapy. These patients were older than the cohort average (44 and 53 years), with one male and one female, but the limited numbers preclude any inferences about this. Both had switched from a previous DMT (glatiramer acetate and dimethyl fumarate) but both screened after the target 120 days (129 and 136 days respectively). Neither patient had visual symptoms. In one case, there was minimal intraretinal fluid and no discernible visual impact hence this was managed expectantly with continuation of fingolimod and had resolved spontaneously during follow-up over two months. There have been no visual sequelae documented since but, interestingly, the patient was diagnosed with type 2 diabetes within six months after this finding. The second patient had significant unilateral macular oedema and measurable acuity reduction (6/12+2) despite a lack of visual symptoms. Fingolimod was stopped immediately and the OCT changes had resolved within three months.
The majority of patients were switching to fingolimod from natalizumab, due to progressive multifocal leukoencephalopathy (PML) risk with the latter, but fingolimod was being used first-line in a significant proportion as outlined in Figure 2.
Other safety considerations
Baseline ECG abnormalities were documented in five patients (12.5%). These included sinus bradycardia (n=2) and incomplete RBBB (n=3). No patients required extended admission (overnight or otherwise) for cardiac abnormalities during fingolimod initiation.
Documented infections occurred in two patients (5%) within the first year of fingolimod treatment. One patient had recurrent conjunctivitis, herpes labialis and scalp papillomata – the scalp lesions were biopsied and shown to be viral. The patient commenced prophylactic acyclovir but was subsequently switched to alemtuzumab due to recurrent infections. The other patient had VZV (shingles) which resolved without significant sequelae despite continued fingolimod use.
Basal cell carcinoma occurred in one patient (2.5%) and this was excised but fingolimod continued. No other neoplasias were documented in this cohort.
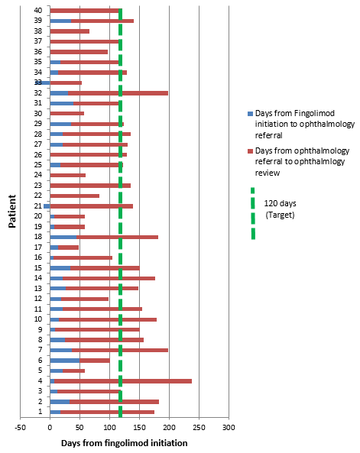
Figure 1: Duration between fingolimod initiation and opthalmology review for FAME
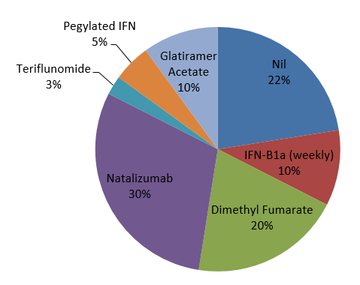
Figure 2: DMT use prior to fingolimod
Conclusions
All patients starting fingolimod in our centre were screened for macular oedema but the majority waited beyond the recommended three to four months. Whilst mean duration between first dose and ophthalmology assessment was potentially appropriate at 124.8 days, 25 patients (62.5%) waited beyond four months (120 days) including two patients found to have macular oedema, albeit without any symptomatic impact. Delays include both the time taken to refer for macular screening after fingolimod initiation and the wait for outpatient ophthalmology, the latter being the greater but arguably less modifiable factor. Other safety issues were considered, including cardiac, infections and neoplasia and were in keeping with expected rates.
Recommendations for future practice:
- Higher risk patients continue to be identified and pre-screened for macular oedema
- Referral to ophthalmology is made on day of discharge (in conjunction with discharge letter)
- Standard ophthalmology referral including statement ‘must be seen within four months’
- Consider ‘pre-referrals’ for patients planning to start fingolimod
- Potentially inappropriate if patients do not start treatment as expected
- ?‘OCT only’ assessment by ophthalmology technician
- Avoids need for consultant appointment
- Ensure clear advice to patient to seek medical attention if visual symptoms occur
- Patient given fingolimod information sheet already
- ?need for first-dose admission for cardiac monitoring
The findings of this audit will be presented to the local MS team to decide on interventions and will be re-audited thereafter, once the interventions are implemented.
References
- Ingwersen J, Aktas O, Kuery P, Kieseier B, Boyko A, Hartung HP. Fingolimod in multiple sclerosis: Mechanisms of action and clinical efficacy. Clin. Immunol. 2012; 142: 15–24.
- Scottish Medicines Consortium. Fingolimod (as hydrochloride), 0.5mg hard capsules (Gilenya). 2012. https://www.scottishmedicines....
- National Institute for Health and Care Excellence. Fingolimod for the treatment of highly active relapsing-remitting multipe slcerosis. 2012. https://www.nice.org.uk/guidan...
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–15.
- Kappos L, Radue E-W, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401.
- Calabresi P a, Radue E-W, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 545–56.
- Subei AM, Cohen JA. Sphingosine 1-Phosphate Receptor Modulators in Multiple Sclerosis. CNS Drugs. 2015; 29: 565–75.
- Zarbin MA, Jampol LM, Jager RD, et al. Ophthalmic evaluations in clinical studies of fingolimod (FTY720) in multiple sclerosis. Ophthalmology 2013; 120: 1432–9.
- Novartis Pharmaceuticals UK Ltd. Gilenya 0.5mg hard capsules – Summary of Product Charcateristics. https://www.medicines.org.uk/E...
Dr Paul Gallagher and Dr Sarah-Jane Martin, Institute of Neurological Sciences, Glasgow
More MS Academy Medication Projects

Encouraging excellence, developing leaders, inspiring change
MS Academy was established in 2016 and in that time has accomplished a huge amount with exciting feedback demonstrating delegates feel inspired and energised along their personal and service development journeys. The various different levels of specialist MS training we offer are dedicated to case-based learning and practical application of cutting edge research.