Improving the safety of natalizumab prescribing at Nottingham University Hospitals
Poster
Introduction
Natalizumab is a monoclonal antibody used in the treatment of highly active relapsing-remitting multiple sclerosis (RRMS). It works by reducing the ability of inflammatory immune cells to attach to and pass through the cell layers lining the blood–brain barrier, and is administered monthly by intravenous infusion. Natalizumab is a relatively safe therapy; however treatment does carry a risk of causing a relatively rare brain infection; progressive multifocal leukoencephalopathy (PML). PML is caused by John Cunningham virus (JCV) – a virus that can is usually kept under control by the immune system, but can lead to PML in the context of immunosuppression. To mitigate against this risk, patients treated with natalizumab are required to have regular MRI brain scans and JCV blood tests.
Anecdotally, there have been ongoing concerns from members of staff over the safety of natalizumab prescribing at Nottingham University Hospitals (NUH), and whether current practice and monitoring is safe and fit for purpose. Following a previous audit, additional safety measures (JCV blood testing of all patients in January and July of every year and uploading of these results onto Notis, our electronic bloods system) have been put into place. Our aim is to see whether these measures have improved compliance with JCV blood testing and to review the safety of our current practice as a whole.
Current practice
NUH has a total of 79 patients on natalizumab. Current practice involves having an MS nurse responsible for maintaining the natalizumab database overall, but each consultant is responsible for their own patients. Each consultant should check the database regularly to see when MRIs are due, with occasional prompts from the MS nurse.
Audit standards
- 100% of high-risk patients (those on Natalizumab for >2years and with high JCV index or previous immunosuppressant treatment) should have had an MRI scan of the brain within the past 6 months.
- 100% of non-high-risk patients should have had an MRI scan of the brain within the past 12 months
- All low-titre JCV and JCV negative patients should have had a JCV blood test within the past 6 months (and all of these results should be available to view on Notis)
Method
The natalizumab database was first updated to ensure that the dates of most recent MRIs and JCV tests were accurate. The database was then audited against the above standards.
Results
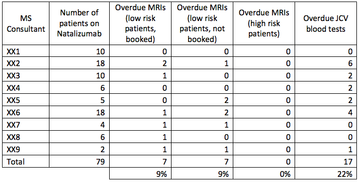
MRIs
A total of 7 patients (roughly 9% of our cohort) have MRIs that are overdue and have not been booked. All of these patients fall into the “low risk” category; however this is still felt to be unacceptable and shows that the current system is not working properly.
A further 7 patients have MRIs that are overdue but have been booked – this could be due to issues with the MRI department or patient availability.
There was some variance amongst consultants, and in general the consultants who had more patients on natalizumab were the ones who appeared to be more likely to have outstanding MRIs to book, this may indicate potential capacity issues and the need for an alternative system.
JCV blood testing
17 patients did not appear to have JCV blood tests within the past 6 months. This has been looked into further and it appears that all of these patients did have blood tests carried out, but the results of these have not been uploaded onto our electronic system.
Discussion
Two out of three audit standards were not met. 82% of low-risk patients had an MRI within the past 12 months (although half of those without an in-date MRI had an MRI booked). 100% of patients had JCV blood tests within the past 6 months, but only 78% of these were available to view on our online system. There were no high risk patients with overdue MRI scans. Having twice yearly JCV testing in all patients does appear to have improved compliance with blood testing, however the fact that these results are not easily available to view is not ideal.
Actions to improve safety
Natalizumab drug chart
Our current Natalizumab drug chart (see Appendix 1) contains boxes to fill in date of last MRI/ JCV status; however these are often not completed. The current drug chart also requires a prescriber to sign every month for each individual infusion, and due to the relatively high number of patients we have on Natalizumab this has led to prescribers signing without checking bloods or MRI status assuming this is addressed by nursing staff.
The Natalizumab drug chart has been redesigned (see Appendix 2) so that it now requires a prescriber to sign once for every 6 infusions (in the majority of our patients this will be every 6 months). It is hoped that by reducing the amount of times the chart needs to be signed, prescribers will be prompted to check and complete JCV and MRI results each time. While this will not be our primary method of ensuring that these are done in time, it is hoped that it will get prescribers into the habit of looking at these and booking MRI scans well in advance.
In the future it is hoped that (as at other trusts) a pharmacist will take over the prescribing and monitoring of Natalizumab. Funding for this is currently not available but a business case has been put forward.
Natalizumab database monitoring
It is acknowledged that the MS nursing team at NUH are overworked and understaffed, and as a result DMT monitoring has understandably been suboptimal to some degree. Because of this, the Natalizumab database and responsibility for monitoring have now been taken over by a Pharmacist. We will re-audit in 6 months to see whether this has made a difference to standard of care.
Conclusion
There is room for improvement with the way we prescribe and monitor Natalizumab at NUH. It is hoped that the above measures will improve practice and patients safety.
Appendix 1
Appendix 2
More MS Academy Medication Projects

Encouraging excellence, developing leaders, inspiring change
MS Academy was established in 2016 and in that time has accomplished a huge amount with exciting feedback demonstrating delegates feel inspired and energised along their personal and service development journeys. The various different levels of specialist MS training we offer are dedicated to case-based learning and practical application of cutting edge research.