Everything a physician needs to know about urinary problems in Parkinson's: Parkinson's Cutting Edge Science conference 2022
KnowledgeOur Parkinson's Cutting Edge Science conference this year, chaired by Dr Emily Henderson, was attended by over 170 Parkinson's specialists spanning Neurology, Old age psychiatry, and Medicine for the elderly, with nurses, therapists and consultants discussing and debating nuanced aspects of Parkinson's treatment, management and care. Each week, we will take a closer look at one of the brilliant sessions.
Neurologist Dr Jalesh Panicker, addressed urinary problems in Parkinson's and 'everything a physician needs to know about them'. Opening with the well-known image from James Parkinson's Essay on the Shaking Palsy, Jalesh noted the detailed examination of motor symptoms given in the essay, and the noticeable lack of content on non-motor symptoms. He shared a few lines at the end of the essay about urinary and faecal incontinence occurring towards the end stage of someones' journey with Parkinson's. However, in an international study into non-motor symptoms through the NMS-Quest study (Martinez-Martin 2007) bladder-related symptoms were most prevalent (fig 1).
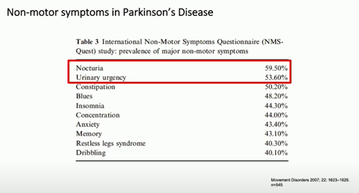
Table 3 of the NMS-Quest study noting prevalences of non-motor symptoms
Jalesh highlighted five key take home messages. His first was that the primary bladder-related issue in Parkinson's is around emptying.
Problems with emptying
There is evidence of worsened motor symptoms correlating with urinary problems such as overactive bladder, voiding difficulties and nocturia, even at the point of diagnosis (Mito et al 2018).
Looking at the different urinary issues that people with Parkinson's can face, Jalesh highlighted that incomplete emptying can often drive other issues such as daytime frequency or nocturia, the most common urinary problem in Parkinson's, and that it is essential to look at how much urine remains in the bladder after emptying to understand if this is a route cause (Batla et al 2016) (fig 2).
He said that nocturia is 'the elephant in the room' in Parkinson's clinics, with 78% and 65% of Parkinson's cohorts in 2 respective studies found to experience it (Smith et al 2015; Romain et al 2015). Commenting on some of the contributing or causal factors in nocturia, he reminded the room that not all bladder problems are located in the bladder - some are caused by changes in the brain. He cited the damage to the parts of the brain responsible for facilitating circadian rhythms in Parkinsonian conditions (Pablo-Fernandez 2018) and noted a pilot study to manage nocturia in Parkinson's using melatonin (Batla 2016).
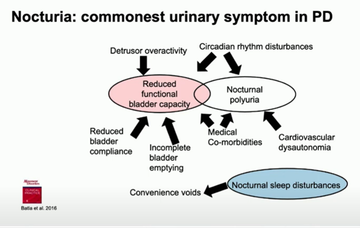
Jalesh presented this slide outlining numerous potential causes of nocturia to consider, impacting different treatment or management approaches
Almost as a side note, Jalesh highlighted the correlation between nocturnal polyuria (classed as when a person voids a third or more of their total urinary volume at night), and falls and fractures. Individuals can experience a higher volume of falls due to waking confused, falling from bed or struggling with dim lighting to visit the toilet, which, particularly if a fracture is incurred, can create a domino-effect of problems and worsened outcomes*.
Jalesh also discussed voiding difficulties briefly, noting these are less commonly experienced by those with Parkinson's, and where they are it is to do with hesitancy or a prolonged or weak stream of urine. Limited detrusor activity, the 'push' to empty when releasing urine, is often apparent in people with Parkinson's but is under-recognised; it is the commonest cause for incomplete emptying in Parkinson's. This is known as a low-pressure, low flow void. In some cases there can be a high-pressure, low flow void, in which case there may be a 'bradykinesia' of the external sphincter, poor relaxation of the internal sphincter, an enlarged prostate or other problems.
Urinary symptoms are progressive
Jalesh urged services to ensure patient initiated follow up or another mechanism is kept in place for individuals with urinary symptoms as generally individuals will experience a worsening of these symptoms as their condition progresses (Uchiyama 2011). This correlation occurred not only in disease progression generally, but also with the worsening of other non-motor symptoms (Barone et al 2009).
Urinary symptoms are usually multifactorial
Jalesh briefly but thoroughly warned the room about assuming all bladder-related dysfunction is owing to a patient's Parkinson's. He presented a clear range of other causes, describing the way they may impact, and how to test for them (figure 3).
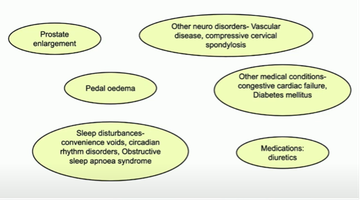
The multiple factors which may cause or contribute to bladder and bowel dysfunction
Not all Parkinsonisms are Parkinson's disease
Jalesh reminded the room of other Parkinsonian conditions including multiple system atrophy (MSA) and noted that bladder symptoms often present early on and a patient with MSA may first present to a urologist. The brand new diagnostic criteria for MSA (Wenning et al 2022) highlights voiding difficulties and unexplained urinary urge incontinence as one of its core clinical features (fig 4). Hoe noted specifically the 'unexplained' element of the latter and suggested a thorough examination of potential causes of this before assuming a diagnosis of MSA.
Jalesh highlighted recent research into the MSA prodrome which suggested, with urinary retention, sexual dysfunction and an abnormal anal sphincter, but no other symtoms, there may be a subset of patients who first present in the base, rather than the brain, beginning in the sacral spinal cord (Panicker 2020).
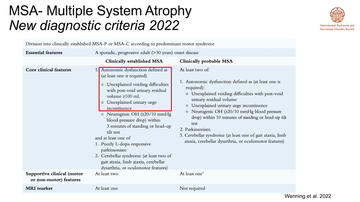
New MSA diagnostic criteria note bladder dysfunction as a primary core clinical feature
There are options for treating urinary dysfunction - but exercise caution
Jalesh rounded out his presentation by highlighting the available treatment options for urinary dysfunction but he was clear that caution must always be exercised. He discussed levodopa and deep brain stimulation and their impact on urinary dysfunction but noted that often urinary symptoms are a secondary consideration which can affect findings (Zong et al 2019, Tabakin 2021).
Treating the bladder itself, Jalesh highlighted the benefits of behavioural therapy for bladder and pelvic floor retraining (Vaughan 2019) and shared a home-based training package that Queen's Square London put together during the pandemic to support people with a neurological condition at home.
He discussed some of the antimuscarinic agents which form the first line of treatment for the neurogenic bladder, and noted that there is very little difference in terms of efficacy amongst the various options and that it is more related to side-effects and how individuals tolerate the treatments.
Jalesh reminded the room that the anticholinergic burden of an individual ought to be considered in light of these medications owing to the cognitive effect that these can have on patients with an overactive bladder (Welk, Richardson, Panicker 2021). He suggested that a younger patient with a lower cognitive risk might be prescribed an antimuscarinic whilst someone over 65 might be better with a beta-receptor agonist, or an antimuscarinic with preferable cognitive safety evidence - and to avoid oxybutynin.
Introducing mirabegron, Jalesh clarified that it is not an antimuscarinic, and shared an open label study in Parkinson's which found significant improvement in some urinary outcome measures and was generally well tolerated (Peyronnet 2018).
Jalesh presented the limited studies using botulinum toxin in Parkinson's patients and noted that generally this has been found to be a safe and effective form of treatment (Vurture et al 2018). However, the impact of botulinum toxin can cause the patient to go into urinary retention and if the patient already has detrusor activity they invariably go into retention, and the patient is counselled about intermittent catheterisation. Because of this, the uptake of botulinum toxin in Parkinson's is reasonably low. If the urinary symptoms are quite significant and as a physician, you can convince the patient of the benefits, then they may choose this treatment, but things like function and dexterity to perform self-catheterisation, if needed, which impact their ability to use this treatment.
Neuromodulation was also presented as an option, such as through stimulating the tibial nerve via percutaneous tibial nerve stimulation (PTNS) (Kabay 2009; Kabay 2016). This can help with symptoms of urgency, frequency and incontinence. Studies examining transcutaneous tibial nerve stimulation )TTNS) have also found positive results in this (Perissinotto 2015) whilst sacral neuromodulation has been found successful on a third of patients with Parkinson's on a short-term basis (Millet 2022). Jalesh also cited a positive study on TTNS in Parkinson's by McClurg et al (2020) but the treatment was not found to be cost-effective or clinically significant enough.
In summary, Jalesh reviewed the key points of his talk but urged people to utilise urinary diaries, highlighting that identifying nocturnal polyuria is not possible without this.
*Education with impact report: 'Supportive skeletons: addressing bone health in Parkinson's to improve outcomes' outlines much of the evidence around worsened outcomes resulting from falls and fractures, and why these are more common in Parkinson's patients.

This meeting is designed and delivered by the Parkinson’s Academy and sponsored by BIAL Pharma. The sponsor has had no input into the educational content of this meeting.
Related articles
'The things you can't get from the books'
Parkinson's Academy, our original and longest running Academy, houses 23 years of inspirational projects, resources, and evidence for improving outcomes for people with Parkinson's. The Academy has a truly collegiate feel and prides itself on delivering 'the things you can't get from books' - a practical learning model which inspires all Neurology Academy courses.