EJS ACT-PD – a clinical trials machine for Parkinson’s Parkinson's Cutting Edge Science conference 2022 18th May 2022
Event reportsOur Parkinson's Cutting Edge Science conference this year, chaired by Dr Emily Henderson, was attended by over 170 Parkinson's specialists spanning Neurology, Old age psychiatry, and Medicine for the elderly, with nurses, therapists and consultants discussing and debating nuanced aspects of Parkinson's treatment, management and care. Each week, we will take a closer look at one of the brilliant sessions.
Part of Cutting Edge Science is to highlight the latest in research opportunities and findings. Our next session did just that, focusing on the brand new multi-arm, multi-stage (MAMS) clinical trial 'EJS ACT-PD'. Neurologist Prof Tom Foltynie, part of the leadership team for the Edmond J. Safra Accelerating Clinical Treatments for Parkinson’s Disease initiative explained how this new form of trial, modelled on the MS Octopus trial, stands to revolutionise the future research around disease-modifying treatment for Parkinson's.
Opening with an overview of research to date, Tom described the frustrations and barriers which have been encountered in trials for Parkinson's treatments historically. He particularly emphasised the time lapse between phase 2 and 3 trials, and the large number of people on a placebo instead of treatment when considered across multiple studies (fig 1).
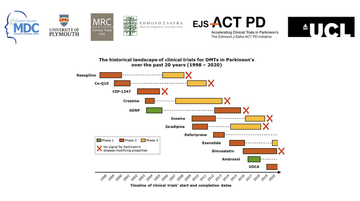
Fig 1: The historical landscape of clinical trials: a slide from Tom's presentation
The MAMs trial format allows for the testing of multiple drugs simultaneously and a placebo group may support findings across multiple drugs, maximising potential benefit to those participating. It also allows for an interim measure where, if there are no signs of efficacy, or signs that the drug is failing in any way, that particular drug can be halted and seamlessly replaced with another drug without any delays in setting up another trail. This 'machine-like' system of recruitment and testing optimises time, funding and opportunities for people with Parkinson's.
Tom shared some of the lessons learned from other multi-arm, multi-stage trials such as those carried out in cancer, highlighting a review where this is all outlined (Zeissler 2020). Tom explained that, to gather more information, Dr Camille Carroll (part of the study leadership) has used a Delphi process to gather early information from stakeholders including people with Parkinson's (including those with previous trial experience), carers, clinical leads and funders of trials in Parkinson's. Tom discussed the findings of the Delphi process which have helped to frame the design of the trial and the working groups which have been established to develop a trial protocol from this information.
Tom then discussed the balancing of priorities amongst those involved and the disparate views that must be understood. He explained the 'consortium' of governance which is working to overcome these and other challenges, to enable all the stakeholders to have their needs met whilst attaining the highest quality of research and best possible evidence and outcomes (fig 2). He discussed each working group in detail, outlining its core functions. He also noted that whilst the working groups have an established membership, the trial continues to seek wider input and involvement.

Fig 2: The EJS ACT-PD Consortium governance structure
Tom outlined the hurdles still to overcome including finalising the trial design and securing the funding. He noted that this MAMs trial is not the only one setting out, with another - 'Path to Prevention' - beginning in the United States under Dr Tanya Simuni. This trial is looking at preventing Parkinson's and will recruit people at risk of developing the condition, whilst EJS ACT-PD is seeking to improve treatment. Tom shared his vision for collaboration both with potential candidates for trials and sharing findings (fig 3).

Figure 3: A vision for collaboration across two MAMs trials in Parkinson's
Finally, Tom highlighted that the UK Parkinson's Clinical Studies Group is being restarted in light of these new trials, and encouraged anyone interested in involvement to make contact.
Related articles
'The things you can't get from the books'
Parkinson's Academy, our original and longest running Academy, houses 23 years of inspirational projects, resources, and evidence for improving outcomes for people with Parkinson's. The Academy has a truly collegiate feel and prides itself on delivering 'the things you can't get from books' - a practical learning model which inspires all Neurology Academy courses.