Before you watch this webinar
Enhancing your learning experience begins with understanding you better. Collecting data enables us to tailor our educational content specifically for our audience. Discover more about how we handle your information in our Privacy Policy.
Event
The Cure Parkinson's Webinar
Cure Parkinson’s is an international funder of research (both preclinical and clinical) focused on disease modification for Parkinson’s. In this webinar session, we will explore the charity’s flagship clinical trials program, called the international Linked Clinical Trials initiative.
This decade-long project involves a committee of 20 leading Parkinson’s experts who gather annually for a two day meeting tasked with prioritising agents that have exhibited evidence for disease modification in Parkinson’s.
This Webinar goes through the history and process of the initiative, and discusses some examples of drugs that have been taken into clinical trials.
Objectives of the webinar:
- A better understanding of the requirements for repurposing agents for Parkinson’s
- Gaps in our knowledge - both scientific and regulatory
- An overview of the clinical trials landscape for agents focused on disease modification in Parkinson’s
CPD accreditation
'The Cure Parkinson's Webinar' has been approved by the Federation of the Royal Colleges of Physicians of the United Kingdom for 1 category 1 (external) CPD credit(s).
Dr Simon Stott | Presentation slides

Summary
The webinar was conducted in association with the Cure Parkinson's Trust. Dr Simon Stott, Director of Research at the organisation, explained how Cure Parkinson's differs from other Parkinson's charities and discussed how current research profile they are supporting.
Cure Parkinson's is UK-based but operates across an international remit, supporting research globally focussed on 'curing' Parkinson's - a large amount of which is looking at disease-modifying treatment for Parkinson's disease.
Founded in 2005 by four gentlemen living with Parkinson's who found that their care was good but that the research aspect was disappointing, the Cure Parkinson's Trust has a specific remit:
'To identify, drive and fund research with a potential to cure Parkinson's'.
Simon explained that, by 'cure', the charity means anything that can 'prevent, slow, stop, reverse or improve Parkinson's long term.'
Simon noted that the organisation also works in understanding progression of Parkinson's including stratification and genetic elements.
Cure Parkinson's supports a gathering of the world's leading clinicians for Parkinson's called the 'International Linked Clinical Trials' committee, which meets annually to discuss ongoing clinical trials and evaluate and consider 15-20 dossiers on new and potential research, aiming to shortlist a handful for Cure Parkinson's to take forward (fig 1).
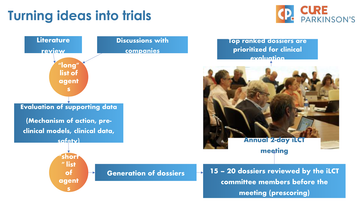
Figure 1: Slide outlining the process used by the iLCT committee
Simon shared in detail the process that the committee goes through, how they review, measure, evaluate and consider the dossiers for consideration. He also highlighted that, whilst the clinicians are the only ones able to vote on which dossiers to take forward, the committee is open to wider stakeholders including others in the voluntary sector and anyone affected by Parkinson's. Those in attendance will be consulted with and asked to share their thoughts and offer questions to inform the decisions made.
Cure Parkinson's is mandated to take forward two to five of these research ideas into clinical trial and they identify trial sites, explore funding opportunities, discuss the concept with wider stakeholders and work with government and Industry to design the trials. The iLCT committee was established 10 years ago, and since then Cure Parkinson's have been responsible for a high volume of trials involving a large number of people with Parkinson's (fig 2).

Figure 2: The iLCT initiative in numbers
Simon then presented some of the trials in more detail, focusing on the completed trials and those expecting results (the latter shown in dark blue on figure 3).
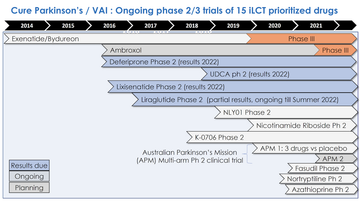
Figure 3: The clinical trials over the years
Simon outlined several studies into a treatment which may be neuroprotective for people with Parkinson's. The treatment, Exenatide, has a growing body of evidence around it, and research that Simon referred (all subcutaneously injectable) include:
- Harkavyi 2008: Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease (now going into phase 3)
- Phase 2 Exenatide study in Stockholm of 60 people with Parkinson's
- Phase 2 Exenatide study in France of 156 participants (early Parkinson's) - results expected this year
- Phase 2 Peptron trial (slow release form) 100 people with Parkinson's
Other research is being carried out to develop oral versions of exenatide such as in diabetes (Meier 2021) and there are trials examining this oral administration for Parkinson's as well:
- GIPD trial Oslo looking at 120 people with Parkinson's (results expected 2024)
- Phase 2 trial (NEURALY) NLY01 trial of 255 participants (results expected 2023)
Simon went on to outline other research, spurred by the diabetes field, into a dual agonist targeting both GLP-1R and GIP receptors. Similar studies have begun, with KP-405 being developed for trials in Parkinson's.
G-Case activation is another line of interest for Cure Parkinson's. G-Case is an enzyme which assists with waste disposal within cells, and around 10% of people with Parkinson's have a variant of the gene GBA resulting in lower levels of G-Case. Ambroxol is a respiratory medication which is being trialled in Parkinson's to activate G-case enzymes. Studies in support of this line of enquiry include:
- Migdalska-Richards 2016 (mouse model) - ambroxol found to 'rescue' models of Parkinson's
- Migdalska-Richards 2017 (non-human primate model)
- Mullin et al 2020, AIM-PD (human, proof-of concept)
Ursodeoxycholic acid (UDCA) is a naturally occurring bile acid in the body currently used to dissolve gallstones and treat liver disease. It has been found to have neuroprotective benefits by acting on mitochondria in models of Parkinson's:
- Rodrigues 1998
- Mortiboys 2013
- Mortiboys 2015
- Abdelkader 2016
- Current clinical trial 'the Up Study' for UCDA in Parkinson's (results expected this year).
Addressing high levels of iron building up in the brain is another area of consideration for research at present, as, although iron levels increase with age, they appear to be higher in people with Parkinson's. It appears that iron chelators may be neuroprotective in models of Parkinson's. The SKY trial is researching 140 participants at present and should present its findings this year.
New trials on the horizon include:
- Immunosuppressive medication azathioprine, usually used in rheumatoid arthritis, being trialled in AZA-PD, led by Dr Caroline Williams-Gray
- Nortriptyline is one of the antidepressants being trialled in the ADepT-PD study, examining whether antidepressants might have a disease-modifying impact on Parkinson's, led by Dr Annette Shrag
Simon highlighted a list of ongoing clinical and pre-clinical work in Parkinson's called The Hope List, developed by Dr Kevin McFarthing and accessible via bit.ly/ParkinsonsHopeList
The webinar then featured a short video from one of Cure Parkinson's patrons, Mike Tindell, followed by Dr Richard Wyse, Director of Clinical Development for Cure Parkinson's who answered questions from the webinar audience.
Our Parkinson's Disease webinars are available on SoundCloud:
soundcloud.com/neurologyacademy
'The things you can't get from the books'
Parkinson's Academy, our original and longest running Academy, houses 23 years of inspirational projects, resources, and evidence for improving outcomes for people with Parkinson's. The Academy has a truly collegiate feel and prides itself on delivering 'the things you can't get from books' - a practical learning model which inspires all Neurology Academy courses.

